
PRE-CLINICAL & CLINICAL STUDIES
PRE-CLINICAL STUDIES OF BriDGE BY THE REGENERATIVE MEDICAL CENTER AT OHIO STATE UNIVERSITY
Our BriDGE Collagen Technology & Products
Our BriDGE Collagen Technology & Products have been extensively studied by leading researchers. One of our studies was conducted by the highly respected Regenerative Medicine Center at the Ohio State University.
Their studies found that:
-
BriDGE is stabilized intact collagen that degrades very slowly;
-
BriDGE collagen matrix attracts, nourishes the patient’s own growth factors;
-
BriDGE collagen matrix stimulates the patient’s own antimicrobial defenses.

BriDGE Before Implant

BriDGE 3 Days Post-Implant
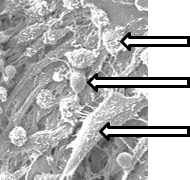
Macrophages
Keratinocytes
New Tissue
BriDGE Collagen Matrix
Accelerates Healing … Prevents Infection
RIGHT AT THE WOUND SITE … 24/7
Another study, a 12-week sheep tendon implant, was conducted by the Surgery and Orthopedic Research Laboratory at the University of New South Wales on our BPMatrix product.
BPMatrix is a collagen patch from our BriDGE collagen processing technology.
The purpose of the study was to characterize the tissue regenerative properties of BPMatrix and compare it to the published results of another collagen implant, Regeneten.
BPMatrix vs Regeneten® Collagen (S&N)
Implants in Sheep Shoulder
BPMatrix Implant at 6 weeks
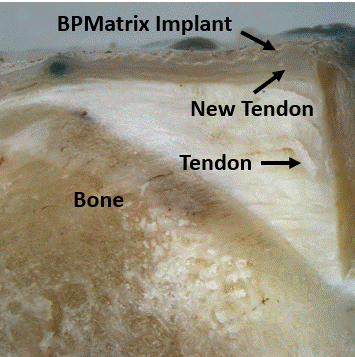
B. Walsh, J Brunelle 2020
Regeneten Implant at 12 weeks
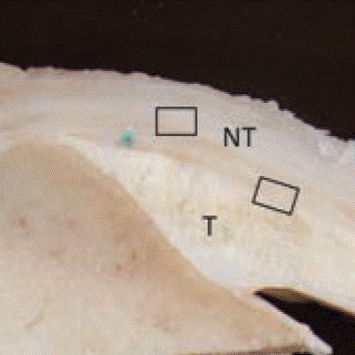
C. Van Kampen, S Arnoczky et al. 2013
Findings by independent lab:
-
BPMatrix implant generated new tendon tissue twice as fast as Regeneten;
-
No inflammatory response to BPMatrix implant;
-
Slowly degrading BPMatrix implant still generating new tendon tissue.
A CLINICAL STUDY FROM 2012
Conducted by Dr. J. Alexander & Dr. D. Yeager
Found on an early version of our products that:
-
For 34 patients with 37 diabetic foot wounds;
-
Overall healing rate at 12 weeks was 75.7%;
-
Median time to closure was 7.0 weeks;
-
All but one wound was treated with a single application of our product.